
Застосування глауконіту. Зелений мінерал, що працює довго на користь ґрунту
Глауконіт — це природний калійвмісний залізо-силікат (шаруватий силікат/філосилікат), який може містити не лише K і Fe, а й Al та інші домішки в кристалічній решітці.

Медицина стоїть на межі нового витка розвитку, і фармакологія — її пульс. Березень 2025 року запам’ятався гучними заявами, науковими проривами та етичними дилемами. Ось найважливіші події, що сколихнули світ фармгалузі.
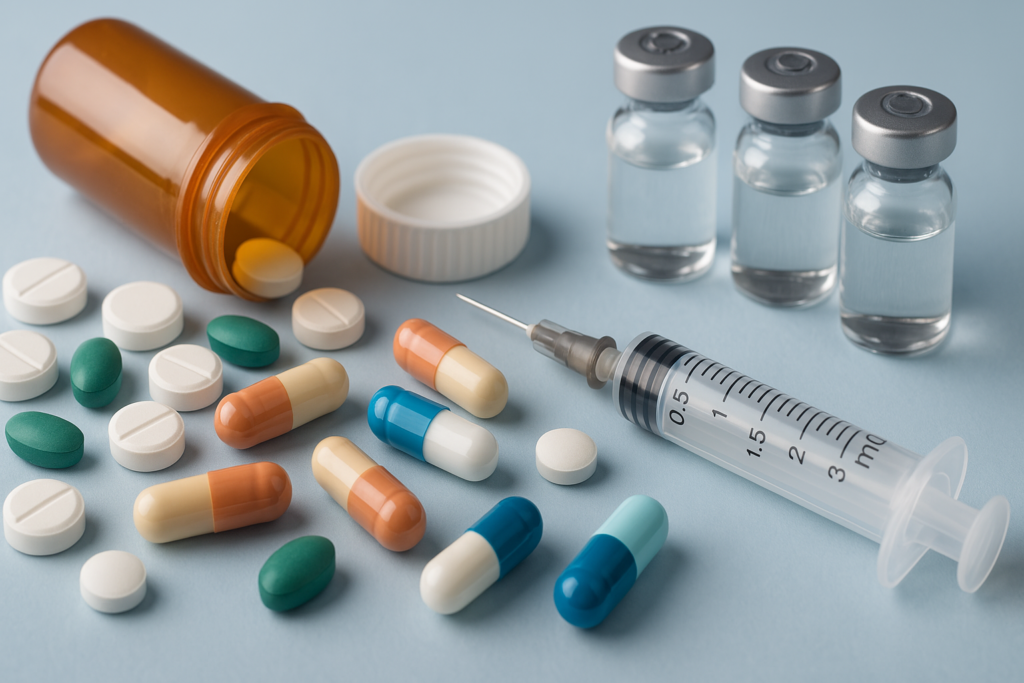
Американська компанія NeuroCore оголосила про успішне завершення третьої фази клінічних досліджень генної терапії для пацієнтів із хворобою Альцгеймера. Препарат NC-β42 показав покращення когнітивних функцій у 62% пацієнтів. Очікується, що заявку на реєстрацію подадуть до FDA вже у квітні. Якщо її схвалять — це буде перший подібний засіб на ринку.
Державне підприємство “Біофарма” підписало угоду з німецьким концерном щодо запуску спільного виробництва вакцин нового покоління на основі mRNA-технологій. Виробництво стартує у червні, з фокусом на вакцини проти грипу, ВПЛ та гепатиту B. Це стратегічний крок у напрямку фармацевтичної незалежності України.
У щорічному звіті ВООЗ зазначено зростання кількості пацієнтів, яким призначають антидепресанти без достатніх підстав — особливо серед молоді у Європі. В організації закликають до впровадження протоколів психіатричного скринінгу перед призначенням психотропних препаратів. Це викликало дискусії про межу між турботою і фармакологізацією емоцій.
Міністерство охорони здоров’я Японії затвердило перший у світі препарат, розроблений з використанням технології CRISPR-Cas9 — EditCellin, для лікування рідкісної форми анемії. Це відкриває нові горизонти для редагування генів у терапевтичній практиці.
У березні аналітики повідомили про 15% зростання інвестицій у розробку фітопрепаратів. Особливу увагу привертає куркумін, канабіноїди та ашваганда. Очікується, що в 2025-2026 роках з’являться нові стандартизовані препарати на основі цих речовин.
Фармакологія швидко змінюється: від молекул до мікроскопічних ножиць, від хімії до біоінженерії. Але за кожною пігулкою стоїть запитання — чи вона справді лікує, чи просто заспокоює страх? У березні ми побачили і надію, і пересторогу. Час не лише ковтати, а й ставити питання.

Глауконіт — це природний калійвмісний залізо-силікат (шаруватий силікат/філосилікат), який може містити не лише K і Fe, а й Al та інші домішки в кристалічній решітці.

Екологізація агро — це не мода, а реакція на факт того, що надлишкові нутрієнти стають джерелом забруднення ґрунтів і вод, втрати біорізноманіття

Дигестат з біовугіллям і глауконітом — інноваційний органо-мінеральний композит для зменшення втрат поживних елементів, пролонгованого живлення рослин і підвищення родючості ґрунтів.